- Adolescent and Adult Patients
- Younger Patients
- Safety
- Product Features
- Younger Patients
For children under 12 years of age BLEED CONTROL AND PROTECTION
ZERO spontaneous bleeds.1*
More spontaneous possibilities.
Ever wonder what life would be like for a child with ZERO spontaneous bleeds? Explore the possibilities with IXINITY®.
Use IXINITY as prophylaxis for bleed control before a child needs it,1 and discover the confidence that comes from proven bleed protection.

* Previously treated patients (PTPs) receiving routine prophylactic treatment of 35 IU/kg to 75 IU/kg of IXINITY once to twice weekly demonstrated a median (interquartile range [IQR]) annualized spontaneous bleeding rate (AsBR) of 0, and a median total annualized bleeding rate (ABR) of 0.86. IQR (range: 0-1.96) (N=21).1-3
IXINITY resolves most bleeds with 1 infusion
In a clinical trial1:
of bleeds were resolved with zero to two infusions
of patients rated bleed control as “excellent” or “good” for bleeds needing treatment1†
†A total of 52 bleeding episodes were treated with IXINITY.1
IXINITY provides predictable bleed control for children’s active, unpredictable life.

Higher recovery may allow lower doses.
Incremental Recovery: The increase in plasma concentration per IU/kg of factor administered.2 Plasma-derived factor products have an incremental recovery close to 1 or 100%.2,3
Selected Important Safety Information
Your body may form inhibitors to IXINITY. An inhibitor is part of the body’s defense system. If you develop inhibitors, it may prevent IXINITY from working properly. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for development of inhibitors to IXINITY.
How recovery impacts infusion dose.§
Hypothetical example: Shannon’s son Kevin weighs 65 lbs (about 30 kg), and needs a 50% factor IX increase (correction).
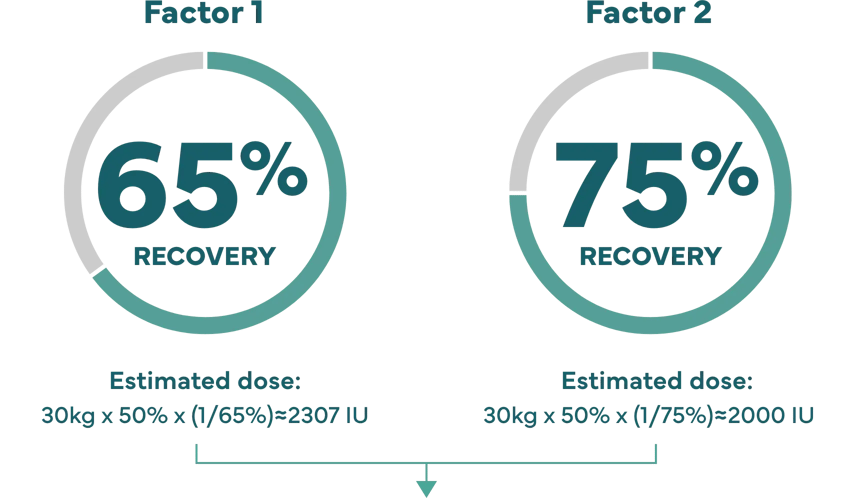
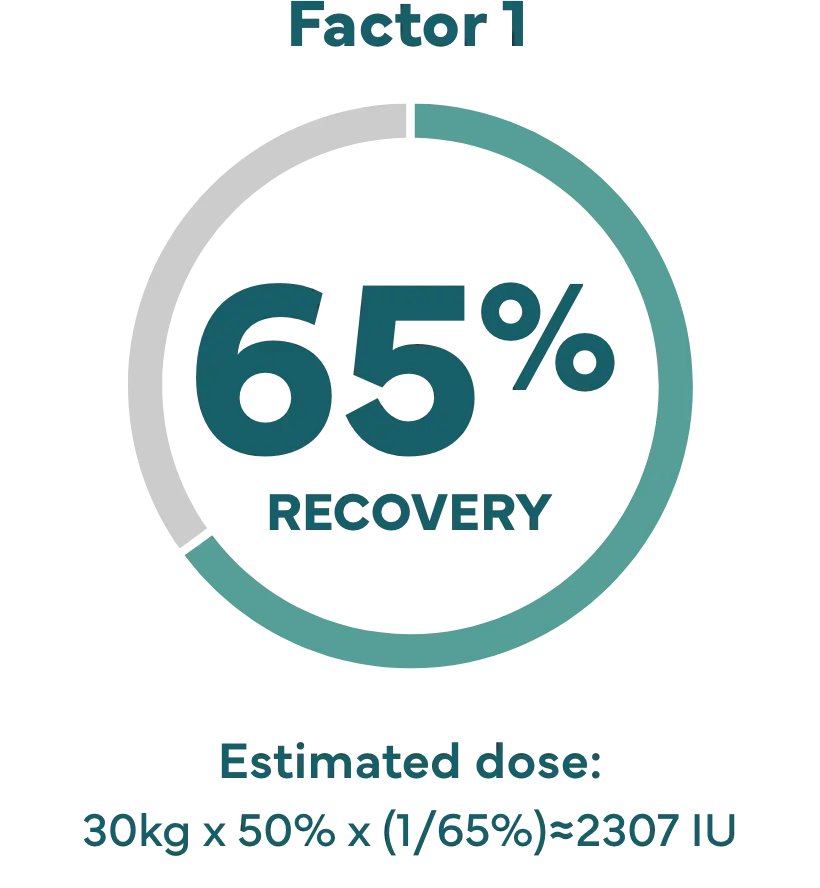
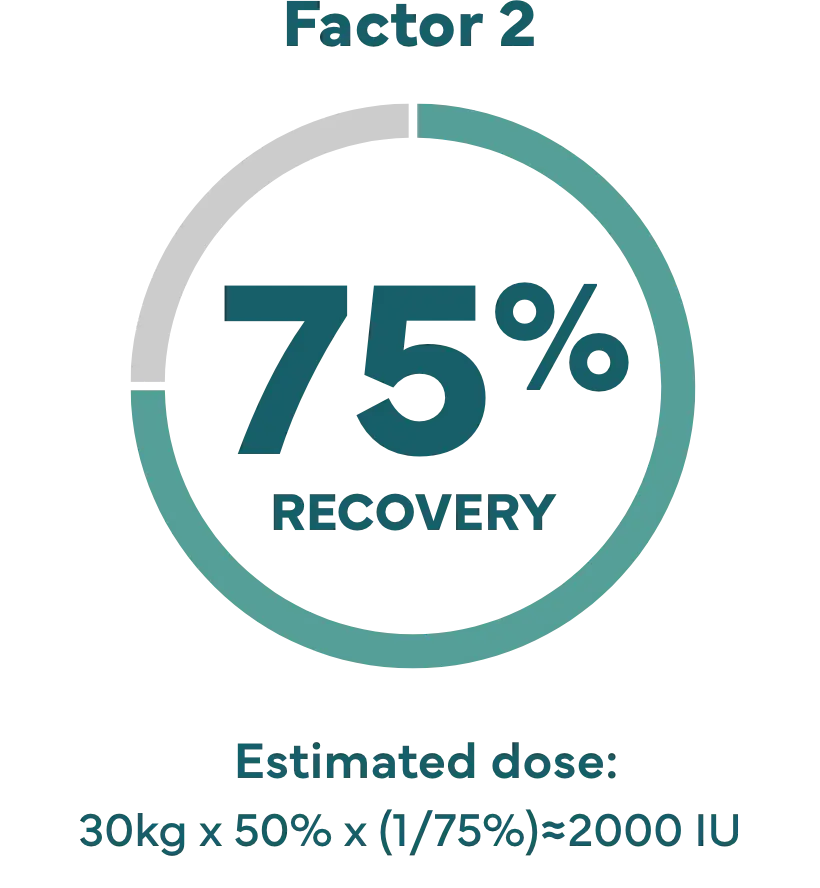
Dose (IU)=body weight x desired factor IX increase x (1/recovery).1
‡ IXINITY recovery is an average based on lab tests of patients in the clinical study.1 Because each child’s actual recovery may be different, their dose may be different, too. Speak with your healthcare professional about the exact dose for them.
§ Description is not of an actual patient. Individual dose will vary. Patient with baseline factor level of <1%.

Peak factor level: Point when the highest concentration of factor in the blood is reached.4 Matching peak factor level timing with peak activity level may help support an active lifestyle.
Terminal half-life: Time it takes for the drug’s activity to decrease by half after factor has been distributed throughout the body. The longer the terminal half-life, the longer factor is active in your system.4
50% of peak factor level remains after 16.3 hours
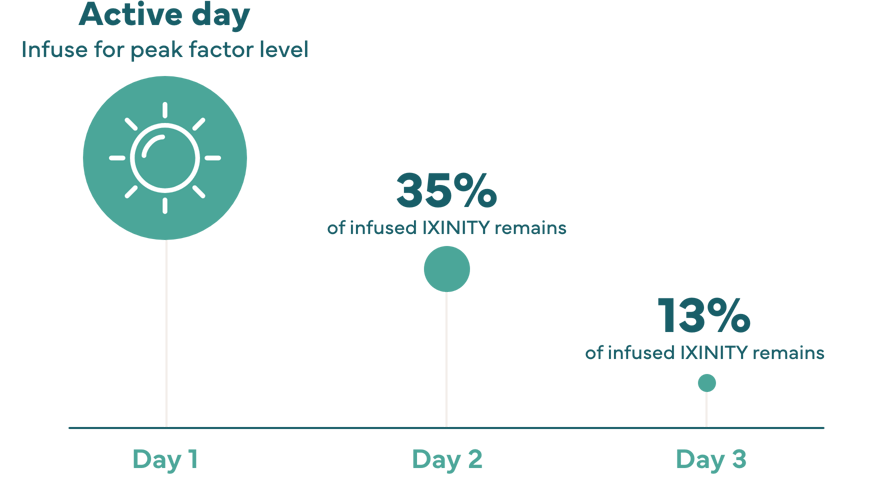
Individual response may very; therefore, number of days between dosing may very.
Questions? Connect with a Hemophilia Territory Manager.
References: 1. IXINITY [coagulation factor IX (recombinant)]. Prescribing information. Chicago, IL: Medexus Pharma, Inc.; March 2024. 2. Björkman S. A commentary on the differences in pharmacokinetics between recombinant and plasma-derived factor IX and their implications for dosing. Haemophilia. 2011;17:179-184. 3. Roth DA, Kessler CM, Pasi KJ, Rup B, Courter SG, Tubridy KL. Human recombinant factor IX: safety and efficacy studies in hemophilia B patients previously treated with plasma-derived factor IX concentrates. Blood. 2001;98(13):3600-3606. 4. Tracy TS. Pharmacokinetics. In: Craig CF, Stitzel RE. Modern Pharmacology With Clinical Applications. 6th ed. Hagerstown, MD: Lippincott Williams & Wilkins; 2004.

